Melanoma Monitoring: The Power of Liquid Biopsy and ctDNA
The treatment of patients with melanoma has seen tremendous success with targeted agents such as BRAF/MEK inhibitors and ICIs for increased survival of advanced-stage melanoma patients. However, a fair number of patients fail to respond or develop resistance. This has drawn much attention to liquid biopsy, especially the detection of `circulating tumor DNA (ctDNA), i.e., noninvasive and dynamic applications for monitoring disease evolution and guiding personalized treatment decisions. ctDNA is a variant of cell-free DNA (cfDNA), which consists of tiny fragments of DNA shed by tumor cells into the bloodstream. Mutations in the genome constitute a type of mutational signature for tumors. A tumor's negative mutational profile will contain alterations that define its aggressive nature. This is a clear-cut definition for a mutational establishment in melanoma consisting of mutations in BRAF, NRAS, NF1, or KIT. Among these, the predominant one is the BRAF V600E mutation, which is also frequently used as a biomarker to track disease progression and guide therapy.
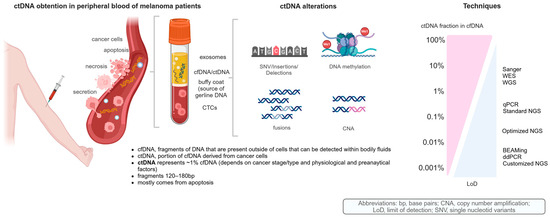
Figure 01: ctDNA in melanoma patients: tumor-derived components in peripheral blood, DNA-based alterations, and analytical sensitivity from current techniques for ctDNA analysis.
The presence of ctDNA is detected through a few extremely powerful methods such as ddPCR, BEAMing, and NGS. In particular, ddPCR seems to provide an excellent tool for the quantification of specific and known mutations such as BRAF and NRAS, while the new age—and broad—NGS panel, including but not limited to Guardant360 and FoundationOne Liquid CDx, profiles many genes associated with cancer formation. Hence, ctDNA enables various clinical applications in melanoma. After surgery for resected high-risk disease (stages IIB–IV), the detection of ctDNA correlated with MRD and a higher likelihood of recurrence. In metastatic melanoma, ctDNA is employed to track response to treatment, differentiate actual progression from pseudo-progression, and identify resistance mechanisms as they arise. A decline in ctDNA levels during ICI therapy has been associated with good outcomes, whereas persistent ctDNA elevation has been linked to poor outcomes.
Yet, ctDNA analysis has some barriers. It has less efficacy in diseases confined to the brain as DNA release is hindered by the blood–brain barrier. Also, ctDNA may be of very low concentration in early-stage melanoma and is hard to detect. Accuracy is also influenced by the technical aspects of ctDNA analysis, such as the type of blood collection tube used, the total time elapsed after blood collection before processing, and so on. Custom ctDNA panels like Signatera™ and RaDaR™ offer greater sensitivity by testing for DNA alterations specific to each patient's tumor profile; however, these tests require more effort and cost. It is believed that tandem use of other biomarkers may boost predictive strength as this field evolves—tumor mutational burden, for example, or markers of an ongoing immune response. Therefore, ctDNA testing will surely be an invaluable component of making informed decisions on individualized care of melanoma patients and give clinicians the ability to make timelier treatment decisions.
Reference
1. Martínez-Vila C, Teixido C, Aya F, Martín R, González-Navarro EA, Alos L, Castrejon N, Arance A. Detection of Circulating Tumor DNA in Liquid Biopsy: Current Techniques and Potential Applications in Melanoma. International Journal of Molecular Sciences. 2025 Jan 20;26(2):861.
Image reference
1. MDPI, https://www.mdpi.com/ijms/ijms-26-00861/article_deploy/html/images/ijms-26-00861-g002-550.jpg:

Comments
Post a Comment