REVISITING LIFE’S ORIGINS: EVIDENCE FOR AN EARLIER EMERGENCE ON EARTH
The question of how life began on Earth has fascinated scientists, philosophers, and curious minds for centuries. From ancient myths to modern scientific inquiry, the origin of life remains one of the most profound and complex mysteries. Among the many theories proposed, the primordial soup theory has stood out as a compelling explanation for how life might have emerged from simple chemical compounds in Earth's early environment. The primordial soup theory suggests that life began in a "soup" of organic molecules, formed in Earth's ancient oceans or ponds, where energy from lightning, volcanic activity, or ultraviolet radiation triggered chemical reactions. These reactions, over millions of years, could have led to the formation of more complex molecules, eventually giving rise to the first living organisms. In 1952, a groundbreaking experiment by Stanley Miller and Harold Urey brought the primordial soup theory to life. By simulating the conditions of early Earth, complete with a mixture of water, methane, ammonia, and hydrogen, along with electrical sparks to mimic lightning—they successfully synthesized amino acids, the fundamental building blocks of proteins. This experiment, now famously known as the Miller-Urey experiment, provided the first experimental evidence that life's essential molecules could arise from non-living matter under plausible early Earth conditions.
 |
| Miller-Urey Experiment |
However, Miller-Urey experiment did not explain how this primordial soup got compartmentalized, allowing for cellular organization and formation of cells. The organic molecules should be compartmentalized in hollow structures which act as chemical microreactors leading to the formation of more complex organic compounds. Over the years, number of mechanisms had been identified which may led to the formation of organic, mineral or mineral-organic membranes which can allow for compartmentalization of organic molecules such as formation of vesicles by non phospholipids, amphiphiles and lipids, encapsulation in gas molecules, liposomes formed from lipids delivered by meteoroids, and mineral membranes formed under accepted conditions of early Earth such as in alkaline environments linked to serpentinization (a geological process which involves hydration of ferromagnesian minerals, resulting in formation of hydrogen gas (H2), methane gas (CH4), and other organic compounds), in soda lakes, or in hydrothermal vents.
For a long time, scientists believed that early Earth, especially during the Hadean eon over 4 billion years ago, was too harsh for life to emerge. The prevailing idea was that only during the later Archean eon, when the atmosphere became rich in CO₂, H₂O, and N₂, could the right chemical conditions exist for life’s building blocks to form. However, recent studies suggest that Earth’s environment may have been much more suitable for prebiotic chemistry much earlier than previously thought. Evidence now indicates that by 4.4 billion years ago, liquid water already covered Earth’s silicate-rich crust, triggering chemical reactions like serpentinization, which produced H2, CO and CH4. These gases, along with volcanic lightning and UV radiation, could have driven the formation of organic molecules. This challenges the traditional timeline of life’s emergence, suggesting that the right conditions existed far earlier than assumed.
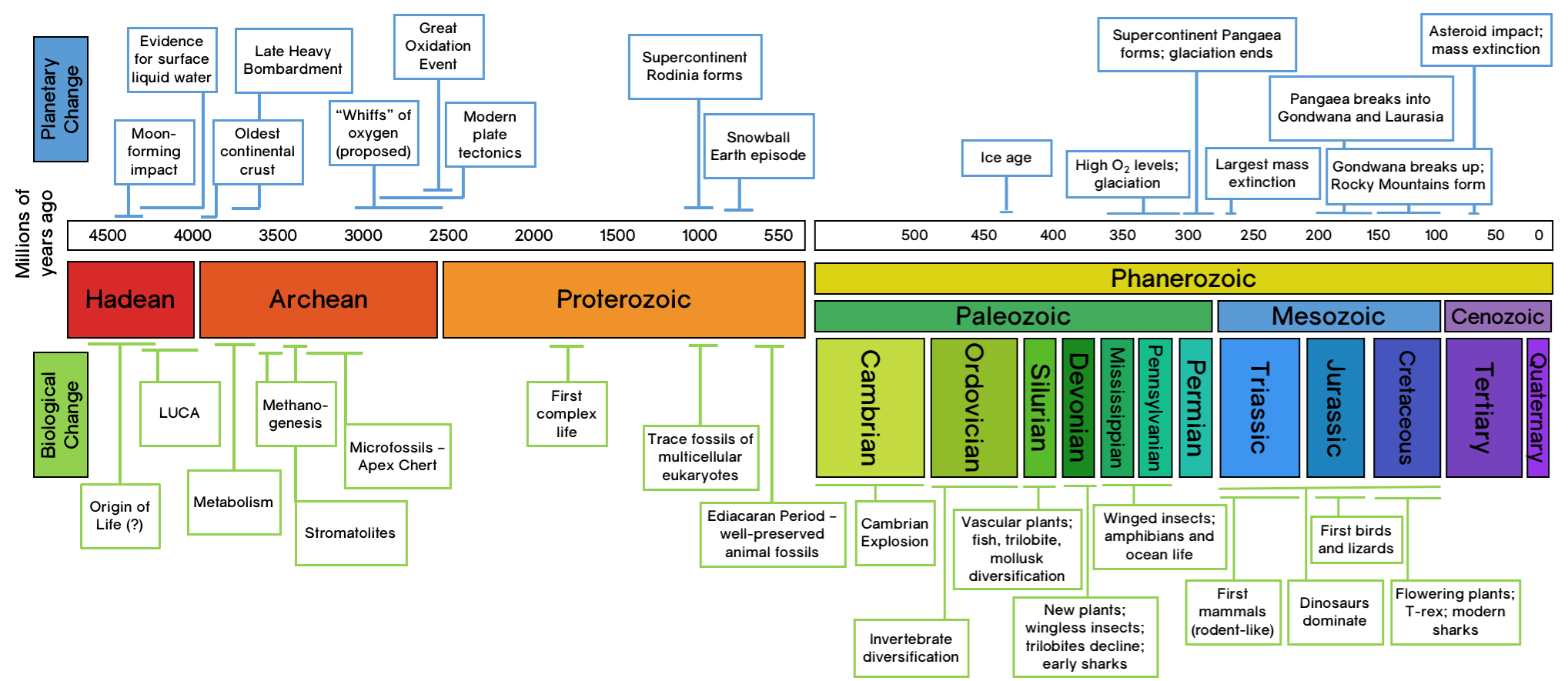 |
| Geological Time Scale |
In a recent experiment, researchers simulated these ancient environments and found that silica-rich surfaces played a crucial role in catalyzing the formation of solid organic matter. The experiment was performed in borosilicate reactors filled with a mixture of reducing gases such as NH3, CH4, N2, water and connected to a generator that supplies energy in the form of electrical discharge. Over the course of two weeks, the clear and highly basic aqueous phase (pH 11.3-11.9) turned strong yellow-brown colour with slight turbidity and drop of alkalinity to pH 10.1 with a brownish organic film gradually forming over the surface during the course of the experiment.
 |
| Changes in the Miller-Urey reactors over the course of 14 days. |
The thickness of the solid organic film (SOF) ranges from 150-3000 nm, composed of fused 50-100 nm sized subunits, and found to have different characteristics based on its location in the reactor with SOF in upper part was less humid and thicker compared to SOF present in lower parts which were thin (<500 nm) and soaked in water due to condensation and capillary action. Field-emission scanning electron microscopy (FE-SEM) showed the presence of micrometer-sized spherical particles directly attached or dispersed on the film, which was formed due to the presence of condensed water.
 |
| FE-SEM images of hollow spaces inside the SOF |
Energy dispersive X-ray (EDX) analysis revealed that the composition of spheres is similar to organic film but with high silicon levels. Morphology was found to be smooth and attached to SOF, with some collapsed which suggests hollow space inside. The internal structure of the spheres are examined by Focused Ion Beam (FIB) sectioning and FE-SEM imaging which revealed that the majority of spheres are hollow with varying internal diameters and thickness. The shrunken version showed wrinkled surfaces which resembled the surfaces obtained by biological origin. Since these spheres allowed compartmentalization of liquids, gases and prebiotic molecules similar to biological cells, these spheres were referred to as protocells.
 |
| FE-SEM images of SOF (B) and spherical structures (C,E-J), (E-F) Images of collapsed spherical structures, (H-J) Images of spheres of varying inner diameter and thickness. |
Apart from spherical particles, other shapes were also observed which ranges from hundred nanometers to several micrometers in length and includes caterpillar-like and polyp-like morphologies. EDX analysis revealed that their composition has higher silicon level compared to surrounding SOF. The cross-section FE-SEM analysis revealed a hollow space which is always connected to a hollow opening in the organic film from which it is hanging and these small openings allow for the growth of these structures or biomorphs by allowing the flow of precursors. These biomorphs appear to grow from the attachment point into the aqueous phase. Some of the larger biomorphs have small openings at the end which may act as the vent for the escape of gases or liquids.
 |
| FE-SEM images of caterpillar-like and polyp-like biomorphs |
Chemical characterization of the SOF was done by comparative analysis of film formed in presence and absence of water. It was observed the SOF formed only in the presence of water, and only a sticky, dark oil was formed in the absence of water. FT-IR analysis revealed presence of α-aminonitriles in water-free experiment with dark oil having basic composition of hydrogen cyanide analogs, acetonitrile, and other isonitriles which can slow some degree of polymerization. FT-IR spectra also showed peaks corresponding to the stretching vibrations of functional groups C≡N, C=N, CH, and NH. On FT-IR analysis of SOF obtained in presence of water, the peak corresponding to stretching vibration of C≡N completely disappears which suggests the conversion of HCN ti progressive ladder polymers and oxygenated chains. The peaks for C≡N and N=C stretching vibrations only appear in water-free experiment suggesting HCN dimers and few longer oligomers being the basic structures.
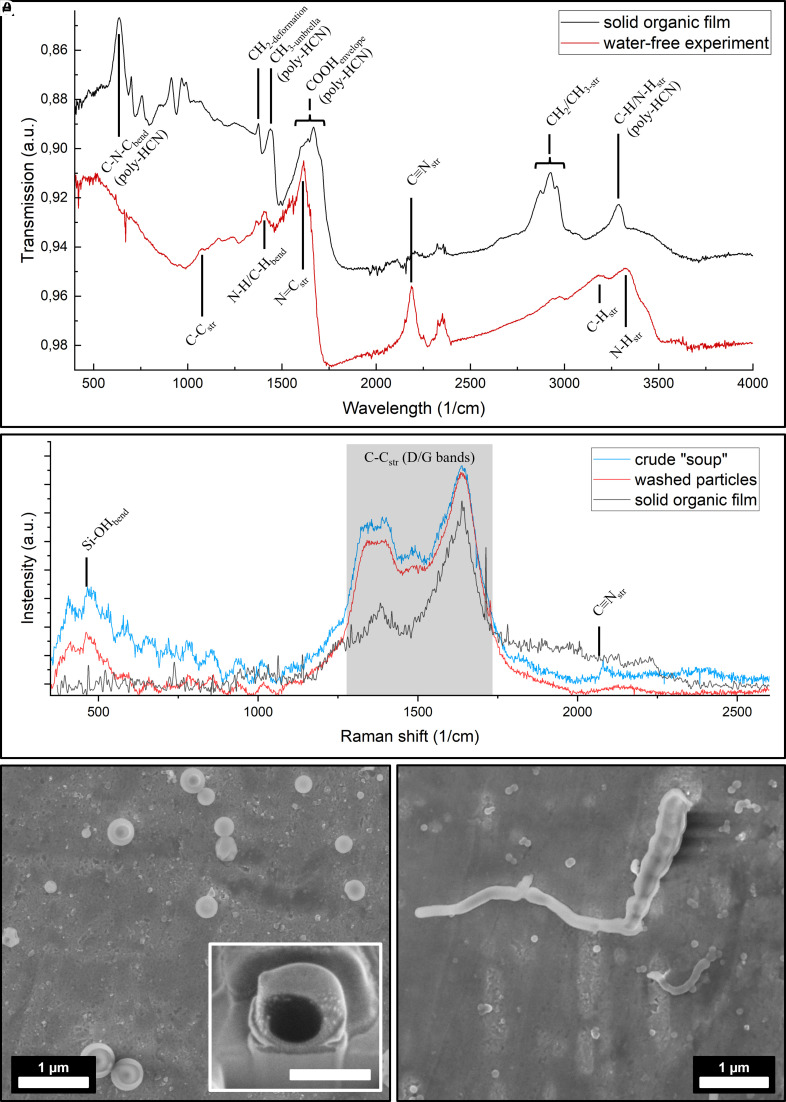 |
| (A-B) FT-IR and Raman spectra of SOF and dark oil. (C-D) FE-SEM images of stably dispersed particles in the soup. |
The peaks corresponding to stretching vibrations of CH in CH2 and CH3 and N-H which was observed in dark oil got reduced to a single intense peak in SOF formed in presence of water. Single band at 1610 cm-1 in the water-free experiment transforms into a complex envelope containing C=N and C=O vibrations suggesting the polymerization of HCN into a variety of polymers as well as formation of new molecular species which were not observed in water-free experiments. Peaks in SOF also showed the presence of carboxylic acids in the form of dimers or more complex structures, CH3 umbrella and CH2 deformation which further suggestd the polymerisation of HCN. Raman spectroscopy also suggested the formation of polymer chains. It was previously proven that SOF formed only in borosilicate glass suggesting the catalytic role of silica in formation of HCN polymers.
At the mesoscopic scale, SOF was found to be composed of fused nanoparticles, with similar particles found to be stably suspended in the slightly turbid solution, which was confirmed by Dynamic light scattering (DLS) and analytical ultracentrifugation (AUC). These nanometer-sized particles (127.3 ± 29.0 nm, 1.24 ± 0.04 g/cm³) likely contributed to SOF formation. Additionally, a second particle species, corresponding to larger spherical biomorphs ranging from several hundred to a few thousand nanometers, was also observed in the solution, as evidenced by DLS and FE-SEM analysis. Liquid-phase Raman spectroscopy of these particles revealed that the dispersed particles and SOF were chemically similar with peaks corresponding to polymerisation. It was also observed that weak C≡N stretching bands present in these particles, aligned with SOF’s IR spectra, which confirmed HCN polymerization. Peaks corresponding to Si-O-Si or Si-OH bending vibrations were also observed in the spectrum. FE-SEM and FIB sectioning also confirmed that these particles obtained after centrifugation were morphologically similar to hollow spherical and worm-like biomorphs. The presence of bands corresponding to silica and polymer in these particles after washing with Milli-Q water suggested that the silica-rich solution is either trapped inside the spheres or became integral part of the spheres. These results suggested that the SOF and the protocells were formed from polymerisation of HCN in the presence of water which resulted in formation of ill-defined poly-HCN nanoparticles.
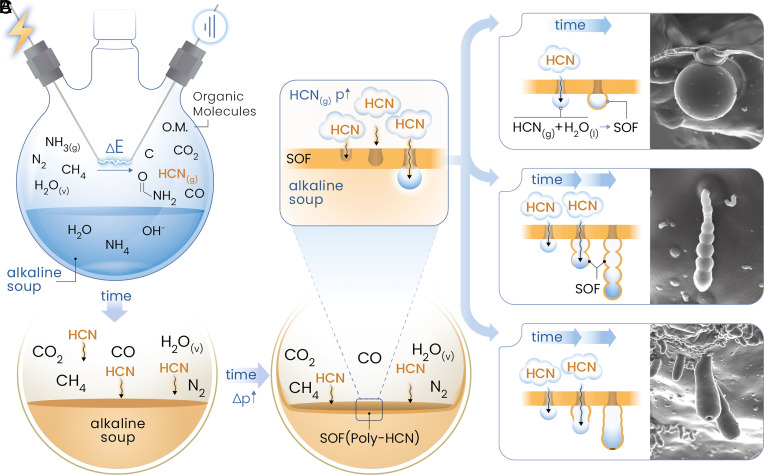 |
| Proposed morphogenesis of the protocells. |
Based on the results, the morphogenesis of poly-HCN biomorphs was proposed which began with hydrogen cyanide formation via electrical discharge and UV radiation, which got converted to formamide or went to direct polymerization in water. It was shown that atmospheric water vapor facilitated the formation of electrically charged HCN-rich nanodroplets, which then got deposited on borosilicate or the water interfaces, which further fused and polymerized into a continuous SOF that occasionally encapsulated organic molecules or droplets. It was suggested that once an initial HCN-polymer layer formed, further interactions with water occurred within the SOF’s porous structure. Unpolymerized HCN derivatives and gaseous byproducts also accumulated in the SOF, which led to bubble formation which mediated their escape into the alkaline medium. At the bubble–water interface, these HCN-rich precursors precipitated as poly-HCN, and thereby encapsulated the bubbles and formed the observed hollow biomorph structures. This precipitation of poly-HCN biomorphs was found to be dependent on factors such as precursor production, HCN partial pressure, and soup pH, which led to the formation of diverse morphologies under varying local conditions. The formation of a single gas bubble beneath the SOF resulted in spherical particles that may remain attached or disperse into the solution. Low HCN concentration or low pH in the environment slowed the polymer precipitation at the bubble interface, which allowed precursor molecules to form secondary bubbles or caterpillar-like and polyp-like structures. The permeability of the poly-HCN shell remained uncertain, but Raman spectra and high densities from AUC of these particles suggested that these protocells would contain liquid rather than gas. These results suggested that these protocells may act as microreactors for prebiotic chemistry as HCN can act as a source of RNA and protein precursors.
These findings suggested that the formation of poly-HCN microreactors, alongside prebiotic organic molecules facilitated by silica, could have transformed early Earth’s waters into large-scale organic chemistry laboratories which would be driven by temperature fluctuations, UV irradiation, and dry-wet cycles to promote increasing chemical complexity. The interaction between silica and small biomolecules may further catalyzed the polymerization, creating biomorphic microreactors that supported molecular evolution. Also variations in atmospheric composition and hydrochemistry would have influenced diverse chemical pathways, some potentially leading to self-sustaining life systems.
In conclusion, these results push back the timeline of life’s emergence from the Archean to Hadean period, suggesting that prebiotic chemistry began much earlier than previously thought. The formation of poly-HCN biomorphs as microreactors for complex organic reactions indicates that Earth’s primitive waters functioned as natural laboratories, driving molecular evolution. By demonstrating that key biochemical processes could have occurred under early Earth's conditions, this research reshapes our understanding of life’s origins and expands the possibilities for life beyond our planet.
REFERENCE:
C. Jenewein, A. Maíz-Sicilia, F. Rull, L. González-Souto, & J.M. García-Ruiz, Concomitant formation of protocells and prebiotic compounds under a plausible early Earth atmosphere, Proc. Natl. Acad. Sci. U.S.A. 122 (2) e2413816122, https://doi.org/10.1073/pnas.2413816122 (2025).
IMAGE SOURCES:
RCS Education, https://d1ymz67w5raq8g.cloudfront.net/Pictures/1024x536/3/9/2/109392_0316eic_feature_origins-of-life_f1_630m.jpg
Britannica, https://cdn.britannica.com/13/244713-050-3751F0F2/Stanley-Miller-Urey-Experiment.jpg
PNAS, https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2413816122/-/DCSupplemental

This comment has been removed by a blog administrator.
ReplyDeleteThis article provides a compelling exploration into the origins of life on Earth, challenging traditional timelines and offering new insights into prebiotic chemistry. The discussion on the Miller-Urey experiment and subsequent advancements in understanding the formation of organic molecules under early Earth conditions is particularly enlightening. The introduction of poly-HCN biomorphs as potential protocells adds a fascinating layer to the theory of life's emergence.
ReplyDelete