BREAKING BLOOD GROUP BARRIERS: MICROBIOTA ENZYMES AS KEY CATALYSTS FOR A TO O CONVERSION
Blood transfusion is an important medical procedure involving transferring blood or blood components from one person (donor) to another (recipient). This procedure is important in various medical situations such as trauma, surgery, chronic diseases, and cancer treatment. The safety and effectiveness of blood transfusion rely heavily on the blood group compatibility between donor and recipient. The most crucial blood group system is the ABO system which is based on the presence or absence of the blood group antigens A and/or B on the surface of erythrocytes (RBC). Correct matching of blood groups is important for blood transfusion since incorrect matching leads to RBC lysis mediated by antibodies against the antigen present in donor RBCs which were transfused and complement present in the plasma of the recipient blood which could be lethal.
Among the four main ABO blood groups: A, B, AB, and, O, only O can be given to any patient hence it is called a universal blood group. Antigen A and B differ from antigen O due to presence of additional sugar moiety α-1,3-linked-N-acetylgalactosamine in the case of A antigen and galactose in B antigen to the core H antigen made of galactose and fucose present in O group. Due to the absence of these additional antigens in the O group, it can be transfused universally to patients with the same rhesus type. However, there is a constant storage of O blood type which is not able to meet emergency demands, and due to this enzymatic conversion of A and B blood groups to O group which involves the removal of the additional sugar moieties proven to be a promising method to meet the constant rising demand of the universal O blood group.
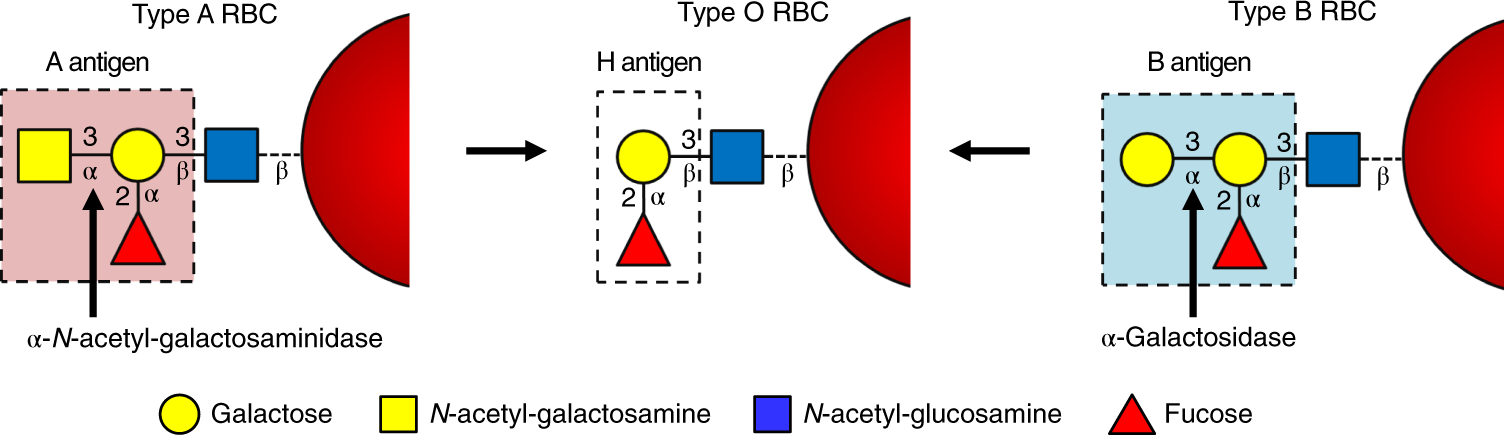 |
| Composition of A, B and O antigens |
The first enzymatic removal of galactose from the B group for conversion to the O group was done using α-galactosidase extracted from green coffee beans. Still, the process required a large amount of the enzyme making the process economically infeasible. The conversion of type A to O is even more challenging due to the presence of several subtypes with three major subtypes A1, A intermediate (Aint), and A2. For better enzyme systems that can convert type A and B groups effectively to O group and whose production can be scaled up, the screening of library of bacteria for both A and B conversion activities was done using tetrasaccharide substrates which identified two families of glycosidases the CAZy (carbohydrate-active enzymes) GH109 α-N-acetylgalactosaminidases and the GH110 α-galactosidases. Screening of metagenomic libraries offers an even more efficient approach for discovering enzyme systems encoded within microorganisms that have not been cultured or even identified. Since the mucin layer of the gut also has these RBC surface antigens, the human gut microbiome may potentially be a source for these glycosidases that can convert type A and B antigens to type O antigen.
 |
| Subtypes of A and B antigen |
The metagenomic library was constructed from large DNA fragments (35-65 Kb) extracted from fecal samples obtained from an AB+ male donor. The library comprises about 80,000 genes and primary screening was done using fluorogenic substrates such as the methylumbelliferyl (MU) α-glycosides of galactose and N-acetyl-galactosamine which was followed by another screening to yield 44 with α-GalNAcase and 166 with α-galactosidase activity. In another screening using antigen A and B tetrasaccharide glycoside substrates, it was found that 11 of the hits contained A antigen cleaving activity, one of which also cleaved B antigen.
The obtained eleven fosmids were sequenced and open reading frames were identified using Metapathways software. Based on the sequence, they are grouped into five clusters. The eight of eleven fosmids obtained were from Bacteroides sp. The cluster A contained GH109 from Bacteroides vulgatus, and cluster B also has a common CAZy protein GH109 from B. vulgatus, while GH109 is the only CAZy protein coded in the cluster containing fosmid obtained from Bacteroides stercoris. Fosmid NO8 obtained from Flavonifractor plautii contains three ORFs within CAZy which included carbohydrate binding molecule CBM32, and two potential glycoside hydrolases GH36 and GH4. In another cluster containing fosmid KO5 from Collinsella sp., A antigen-cleaving activity having GH36 was identified. All these genes were cloned and expressed in E.coli for the characterization of the proteins.
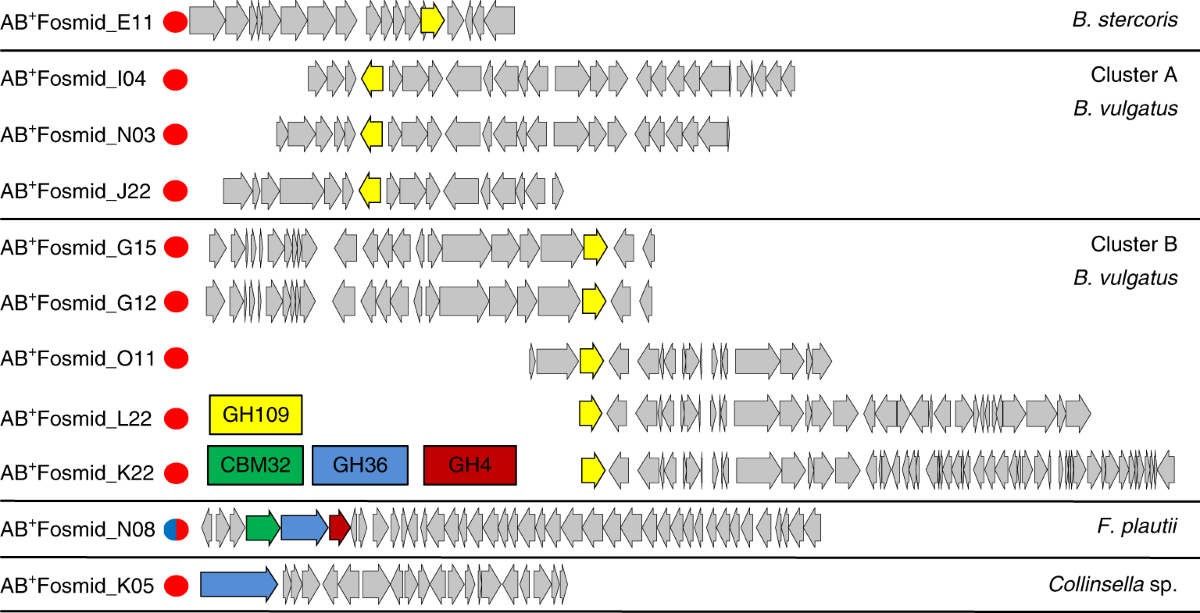 |
| ORFs present in eleven fosmids which were classified into five clusters based on overlapping regions. |
The kinetic parameters of obtained A antigen-cleaving activity having GH109 from different clusters were obtained and was found that they had similar catalytic efficiencies. After this, their ability to remove A antigens from A+ RBCs was tested in the presence of 40 Kd dextran as a crowder to concentrate the enzyme on the surface of RBCs in spin columns which also contained antibodies against A and B antigens and tested the level of retardation of RBCs thus their antigen content, using previously identified GH109 from Elizabethkingia meningosepticum (EmGH109) as control. It was found that in the absence of crowder, none of them were more effective than control. The GH36 protein from fosmid K05 obtained from Collinsella sp. cleaved both methylumbelliferyl (MU) α-glycosides of galactose and A antigen tetrasaccharide, but it was shown to have only limited ability to remove A antigen from RBCs using the same test done for GH109.
Since these enzymes were not as effective as the control, the efficiency of CAZy-related enzymes encoded in the N08 fosmid from F. plautii were tested. When individual purified enzymes were tested against small A and B tetrasaccharide substrates, it was found that only the B antigen substrate was cleaved by FpGH36, and a combination of FpCBM32 and FpGH36 rapidly cleaved the A antigen substrate to the H antigen substrate. Thin-layer chromatography (TLC) showed that FpCBM32 converts A antigen to a more polar ultraviolet-active product and the subsequent addition of FpGH36 removes galactosamine to form H antigen trisaccharide. Mass spectroscopy showed that FpCBM32 is A antigen deacetylase and FpGH36 is a galactosaminidase. This was further confirmed by high-performance anion exchange chromatography with pulsed amperometric detection (HPAE-PAD) analysis. This showed that a combination of these enzymes was able to convert the A antigen to H antigen and these enzymes were named FpGalNAc deacetylase (FpGalNAcDeAc) and FpGalactosaminidase (FpGalNase).
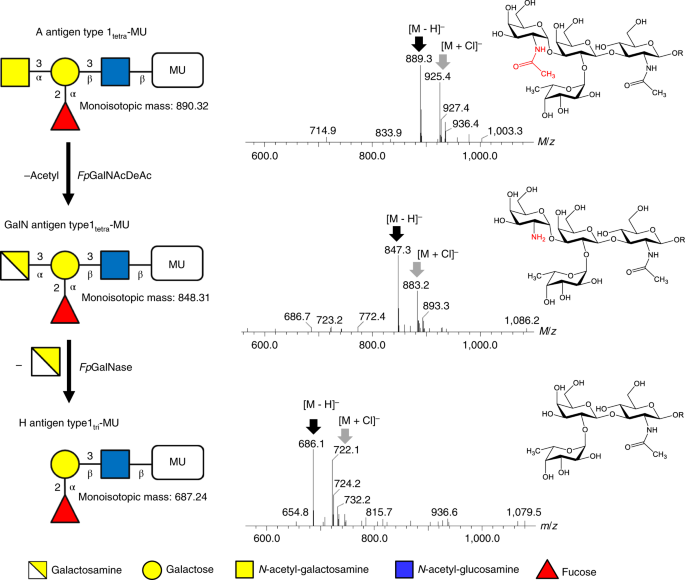 |
| Deacetylation pathway of A antigen cleavage |
Characterization of FpGalNAc deacetylase revealed a 308 amino acid domain at the amino-terminal and 145 amino acid CBM32 near the carboxy-terminal with a linker region between them. It is a metalloenzyme that requires divalent metal ions which were proven by the inhibition of enzyme activity on the addition of EDTA and enhancement of enzyme activity on the addition of Mn2+, Co2+, Ni2+, or Zn2+. It has a broad pH activity with pH 8 being optimum and a narrow substrate specificity to different A subtypes and shorter versions. These properties are ideal for the conversion of the A to O blood group as all different A subtypes can be converted.
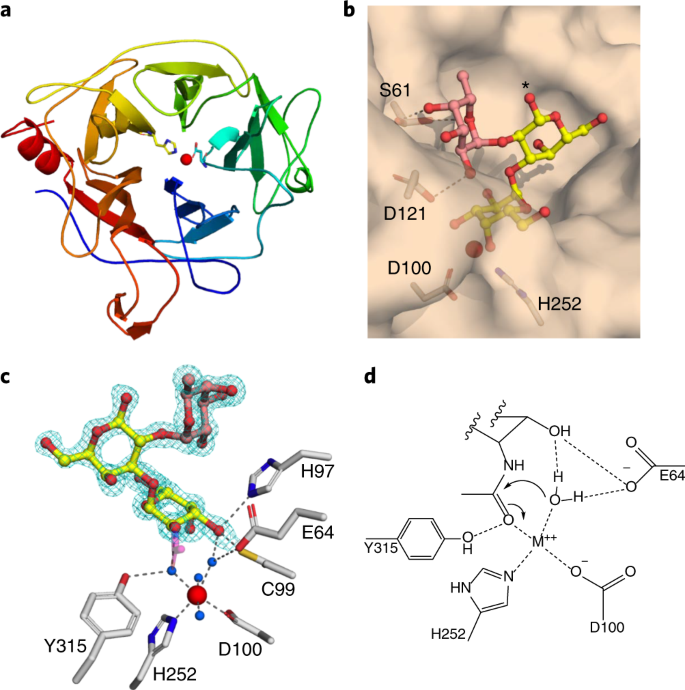
The crystal structure of FpGalNAcDeAc revealed that the catalytic domain adopts a five-fold beta-propeller structure with an active site with divalent cation coordinated by Asp100 and His252. At the base of the active site, the galactose of the A antigen tetrasaccharide forms hydrogen bonds with His97, Glu64, and two of the metal-coordinated waters. Glu64 plays a direct role in the activation of nucleophilic water molecules. Mutation in metal coordinating residues Asp100 and His252 to asparagine and phenylalanine reduced its activity 5000-fold. The polar interactions between the fucose and Ser61 and Asp121 residues explain its specificity to the fucosyl group containing antigens.
Characterization of FpGalNase revealed 390 amino acid catalytic domain with a potential carbohydrate-binding domain at the C terminal. Removal of this domain resulted in decreased efficiency in the cleavage of deacetylated A+ RBCs. the specificity of this enzyme to de-acetylated alpha galactose-configured substrates and pH optimum of 6.5-7 makes it ideal for use in the conversion of blood group in combination with the deacetylase.
 |
| Structure of FpGalNase |
Assessment of the efficiency of this combination of enzymes on RBC conversion, the individual enzymes, and their combination were incubated along with type A+, B+, and O+ RBCs, and the released sugars were analyzed using HPAE-PAD ion chromatogram and it revealed that only galactosamine was released from A antigen and no sugar from B or O antigen only when both enzymes were present, proving the high specificity of the combination of enzymes to A antigen. Further analysis using fluorescence-activated cell sorting (FACS) revealed that the addition of only 5 5 μg ml−1 of the enzyme combination was able to catalyze near complete conversion of A antigen to H antigen even in the absence of macromolecular crowder to localize the enzyme on RBC surface. After conversion, these enzymes can be easily removed by simple centrifugation-based washing procedures but further studies are needed to confirm the complete removal of enzymes that may be recognized by human polyclonal anti-A antibodies which may not be involved in typing which could arise from poorly accessible substrates.
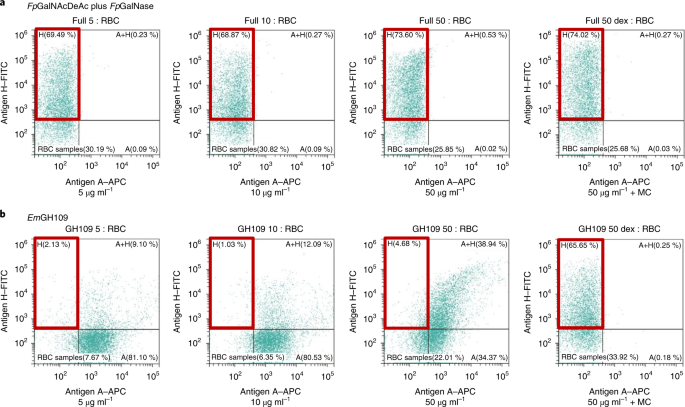 |
| FACS analysis showing conversion near conversion of A to O antigen by the enzyme combination as compared to previously known EmGH109 |
In conclusion, the enzymatic conversion of A and B blood types to the universal O group represents a groundbreaking advancement in healthcare. Enzymes from the human gut microbiome have paved the way for increasing the supply of O-type blood, which is crucial for emergencies where matching blood types can be a challenge. This innovation holds the potential to make life-saving blood transfusions more widely accessible and improve outcomes for patients across the globe, reducing the reliance on exact blood type matches and alleviating shortages in blood supplies.
REFERENCES:
Olsson, M. L., Hill, C. A., de la Vega, H., Liu, Q. P., Stroud, M. R., Valdinocci, J., Moon, S., Clausen, H., & Kruskall, M. S. (2004). Universal red blood cells--enzymatic conversion of blood group A and B antigens. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine, 11(1), 33–39. https://doi.org/10.1016/j.tracli.2003.12.002
Rahfeld, P., Sim, L., Moon, H. et al. An enzymatic pathway in the human gut microbiome that converts A to universal O type blood. Nat Microbiol 4, 1475–1485 (2019). https://doi.org/10.1038/s41564-019-0469-7
IMAGE SOURCES:


Comments
Post a Comment