UNLOCKING NATURE'S SOLUTIONS: MICROBIAL AND ENZYMATIC BREAKDOWN OF SYNTHETIC PLASTIC
Since synthetic polymers are inexpensive, durable, and versatile, they have become a necessary component of modern life. From packaging materials to car parts, apparel, electronics, and medical equipment, plastics have changed various sectors. But the very qualities that lend plastics their utility—like their resistance to deterioration—have also made plastics a serious environmental problem. Concerns regarding the effects of the massive buildup of plastic garbage, especially that which comes from petroleum-derived polymers, on ecosystems and human health have been raised. The problem is exacerbated by the long-lasting nature of these materials, since they can persist in the environment for decades or even centuries without breaking down. Petroleum-based polymers such as polyethylene (PE), polyethylene terephthalate (PET), polyurethane (PU), polystyrene (PS), polypropylene (PP), and polyvinyl chloride (PVC) are well-known for their resilience to natural breakdown processes. This resistance stems mostly from the chemical properties and crystallinity of these polymers, which make them difficult for microbes to degrade. However, in recent years, scientists have discovered and classified bacteria capable of decomposing petropolymers in the laboratory. These findings show promise for developing microbial and enzymatic techniques to degrading plastic waste, perhaps providing a solution to the expanding plastic pollution challenge.
The biodegradability of plastics is intimately related to their chemical structure and physical features, such as molecular weight and crystallinity. Polymers, which are huge molecules made up of repeating units known as monomers, are used to make plastics. These polymers have both regular crystalline and irregular amorphous areas. The crystalline areas are made up of closely packed molecules that give the polymer strength and rigidity, whereas the amorphous portions have loosely distributed molecules that provide flexibility. Highly crystalline polymers, such as polyethylene, have a crystallinity of approximately 95%. This makes them incredibly hard and impact-resistant, but also tough to deteriorate. Polyethylene is widely utilized in products such as plastic bags, bottles, and containers, which are frequently discarded carelessly, resulting in accumulation in landfills and oceans. PET-based polymers, which are extensively used in beverage bottles and food containers, have crystallinity ranging from 30% to 50%. PET, while less crystalline than polyethylene, nevertheless poses substantial obstacles for microbial decomposition, with total disintegration in natural conditions expected to take more than 50 years. The deterioration process in marine environments is much slower due to lower temperatures and limited oxygen availability, often extending the period to hundreds of years.
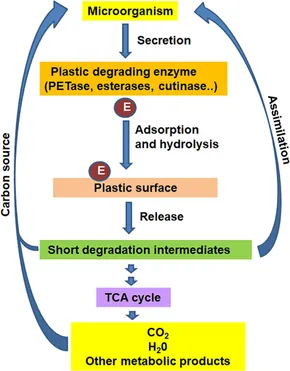
Microorganisms that can degrade synthetic plastics have been isolated from a variety of settings, including soil, marine ecosystems, and even invertebrate digestive systems. These bacteria produce enzymes that can degrade lengthy polymer chains in plastics into smaller monomers, which can subsequently be digested. Enzymatic degradation occurs in two stages: first, the enzyme adsorbs to the polymer's surface, and then the polymer bonds are hydrolyzed or oxidized. The efficiency of this procedure is determined by the accessibility of the polymer surface, which is frequently hampered by the high crystallinity of particular materials. One of the greatest advances in plastic degradation research has been the discovery of enzymes capable of degrading polyethylene terephthalate (PET). These enzymes, such as cutinase and PETase, have been proven to hydrolyze PET's ester linkages, separating it into terephthalic acid and ethylene glycol. The monomers can then be recycled or transformed into biodegradable polymers. Despite these developments, enzymatic breakdown of PET remains slow, and major research is underway to increase the efficiency of these enzymes. Polyurethane (PU), another commonly used material, is likewise a target of microbial breakdown. Products containing polyurethane include foams, coatings, and adhesives. Several bacteria and fungi have been identified as capable of degrading PU, with enzymes like ureases and lipases playing an important role in the process. However, like PET, PU degradation is sometimes incomplete, and the byproducts can be poisonous, making the process less environmentally beneficial. Polyethylene (PE), one of the most common polymers, has considerably more issues. Because of its high crystallinity and hydrophobicity, few microbes have been discovered that can successfully breakdown PE. However, some progress has been achieved, with some bacterial strains demonstrating the ability to partially breakdown PE sheets. Enzymes capable of breaking down the carbon-carbon backbone of PE, which is resistant to hydrolysis, are now being investigated.
The accumulation of synthetic plastics in the environment is a big concern since they are resistant to natural degradation processes. However, developments in microbiological and enzymatic breakdown of petropolymers provide hope for addressing the plastic pollution challenge. Microorganisms capable of degrading plastics have been identified, and the enzymes they produce have been tested for their potential to degrade polymers such as PET, PU, and PE. While obstacles persist, particularly with highly crystalline polymers such as polyethylene, ongoing research into microbial degradation pathways and enzyme engineering may pave the way for more efficient plastic recycling and bioconversion solutions. This, in turn, may lead to a more sustainable approach to handling plastic trash and lowering its environmental impact.
REFERENCE :
Mohanan, N., Montazer, Z., Sharma, P. K., & Levin, D. B. (2020). Microbial and enzymatic degradation of synthetic plastics. Frontiers in Microbiology, 11. https://doi.org/10.3389/fmicb.2020.580709
IMAGE REFERENCE :
https://images-provider.frontiersin.org/api/ipx/w=290&f=webp/https://www.frontiersin.org/files/Articles/580709/fmicb-11-580709-HTML/image_m/fmicb-11-580709-g002.jpg


Comments
Post a Comment